Abstract
Background: B-acute lymphoblastic leukemia (B-ALL) is considered to be the most common malignancy diagnosed in childhood and leading cause of cancer-related death in children. There have been advances in the efficacy of multi-agent chemotherapy and stratification of treatment intensity according to clinical features of the patient, biologic features of leukemia cells, and early response to treatment. These advances herald the promise and challenges of precision medicine strategies that integrate leukemia genomics into contemporary therapy. Despite this progress, there are unmet needs related to the treatment of B-ALL. Among them the incidence varies according to race: African American (AA) children have significantly lower incidence of B-ALL compared to European American (EA). However, studies have shown that AA children have significantly lower survival rates compared to EA children. This disparity is not related to socioeconomic variables, suggesting a molecular basis for the lower survival rates of AA. Here we present a study showing race-specific genetic aberrations involved in leukemogenesis that may contribute to health disparities in B-ALL in AA and EA children.
Aims: In a population of AA and EA pediatric B-ALL patients: 1) Discover the genomic susceptibility architecture at validated loci and determine whether genetic variants confer population-specific risks. 2) Define the landscape of molecular perturbation and genomic alterations. 3) Identify aberrant canonical pathways in leukemogenesis.
Methods: A total of 20 newly diagnosed pediatric patients were utilized in our study (5 AA and 15 EA). Ages ranged between 1 and 18 years. Clinical history, hemogram, flow cytometry, immunophenotypic, morphologic, cytogenetic and prior molecular data were reviewed. None of the patients in our study had a relapse. Median percent cellularity present in the bone marrow aspirates was 100% (70% - 100%). Median percent of blasts was 94.8% (64.5% - 99.0%). Following manufacturer protocol (Qiagen, QIAmp DNA blood kit), 200µl of frozen bone marrow aspirates were used to extract the DNA. Following quality control on a Qubit fluorometer and RNA bioanalyzer, the samples were submitted to a translational genomics core for library preparation and whole-exome sequencing (TruSeq Exome, Illumina). Bioinformatics analysis was performed and genetic variants for each sample were identified.
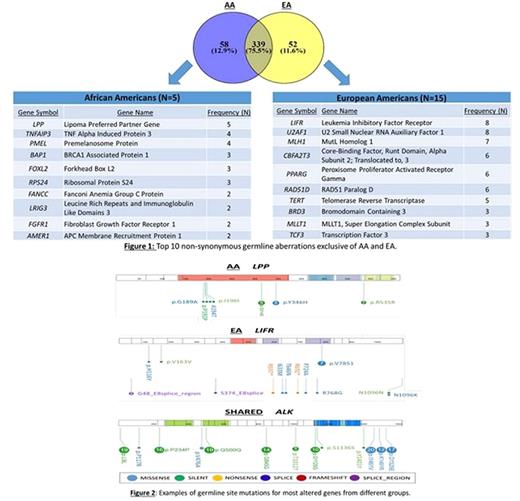
Results: We identified specific germ-line mutations within the most widely accepted cancer-related genes related to B-ALL. Most genetic aberrations (339) were shared between AA and EA, for example those present in the Anaplastic Lymphoma Receptor Tyrosine Kinase (ALK) gene. Some genes with genetic aberrations were specific of AA (58) such as, Lipoma Preferred Partner Gene (LPP) and others specific for EA (52) such as, Leukemia Inhibitory Factor Receptor (LIFR). The ingenuity pathway analysis revealed these genes clustered in race-specific canonical pathways. In AA, the specific pathways were related to telomerase signaling and cancer signaling. In EA, the specific pathways were related to stem cell pluripotency and hereditary cancers. Our findings suggest the value of whole exome sequencing as a tool for development of individual gene signatures and gene scores for AA and EA children afflicted by B-ALL.
Conclusions: Overall, the prevalence of genetic aberrations in B-ALL may contribute to differences in incidences and outcomes between AA and EA children. Aberrant biological networks revealed by our study, provide information on distinguishing genetic aberrations and signaling networks that may be involved in race-specific leukemogenesis. Furthermore, our findings suggest that it may be possible to develop a whole-exome sequencing gene signature in B-ALL to help define a race-specific prognostic assay for B-ALL in bone marrow aspirates. In the future, the biomarker panel derived from this study will help guide risk stratification and treatments based on race and advance the prognosis of B-ALL in AA children. These findings may ultimately impact targeted disease management and contribute to the elimination of the disparate outcomes in B-ALL among AA children.
Funding: Hyundai Hope on Wheels Scholar Grant, UMMC Division of Pediatric Hematology Oncology
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal